5.3: stoichiometry calculations Stoichiometry problem gas chemistry working # 3 stoichiometry mass to mass
Stoichiometric Calculations: An example of doing 2 (out of 3 steps
Stoichiometry, gas stoichiometry Stoichiometric calculations Stoichiometry: mass to mass
Examples of mass to mass stoichiometry problems
Stoichiometry of reactions in solutionStoichiometry formula Libretexts chem stoichiometry equations formulas flowchart calculations bookshelves stoikiometriComposition stoichiometry.
Essay writing serviceDimensional analysis stoichiometry chem conversions Stoichiometry stoichiometric calculations example using stepsMass stoichiometry.

Stoichiometry problem mass chemistry
Chem 101: dimensional analysis – stoichiometry and mass to massStoichiometry problem type 3: mass to mol Stoichiometry: mass to massLecture: stoichiometric calculations.
Stoichiometric calculations: an example of doing 2 (out of 3 stepsStoichiometry stoichiometric calculations Solved n-butane fuel (c_4h_10) is burned with aStoichiometry mass.

Stoichiometry with mass: stoichiometry tutorial part 2
Stoichiometry moles 7o2 o2 h2o equationMore stoichiometric calculations 1. calculate the mass of Stoichiometry with massStoichiometry mass tutorial.
Stoichiometry massStoichiometry practice Week 8 stoichiometry (mass to mass)Burned fuel fraction solved amount stoichiometric butane transcribed text calculate.
.PNG)
Calculations stoichiometry equations stoichiometric solution chemistry chemical balanced flowchart number reactions chem quantitative moles volume formulas calculating reaction problem balancing
Mass stoichiometryStoichiometry calculations stoichiometric equations balanced molarity quantitative chem moles grams solutions formulas molar flowchart substances dilutions converting libretexts given reactants Mass to mass stoichiometryMass stoichiometry problems examples.
Calculate stoichiometric calculations massUsing stoichiometry to determine a formula Stoichiometry igcse chemistry equations three calculation these must memorize heart offComposition stoichiometry lesson.


MORE STOICHIOMETRIC CALCULATIONS 1. Calculate the mass of
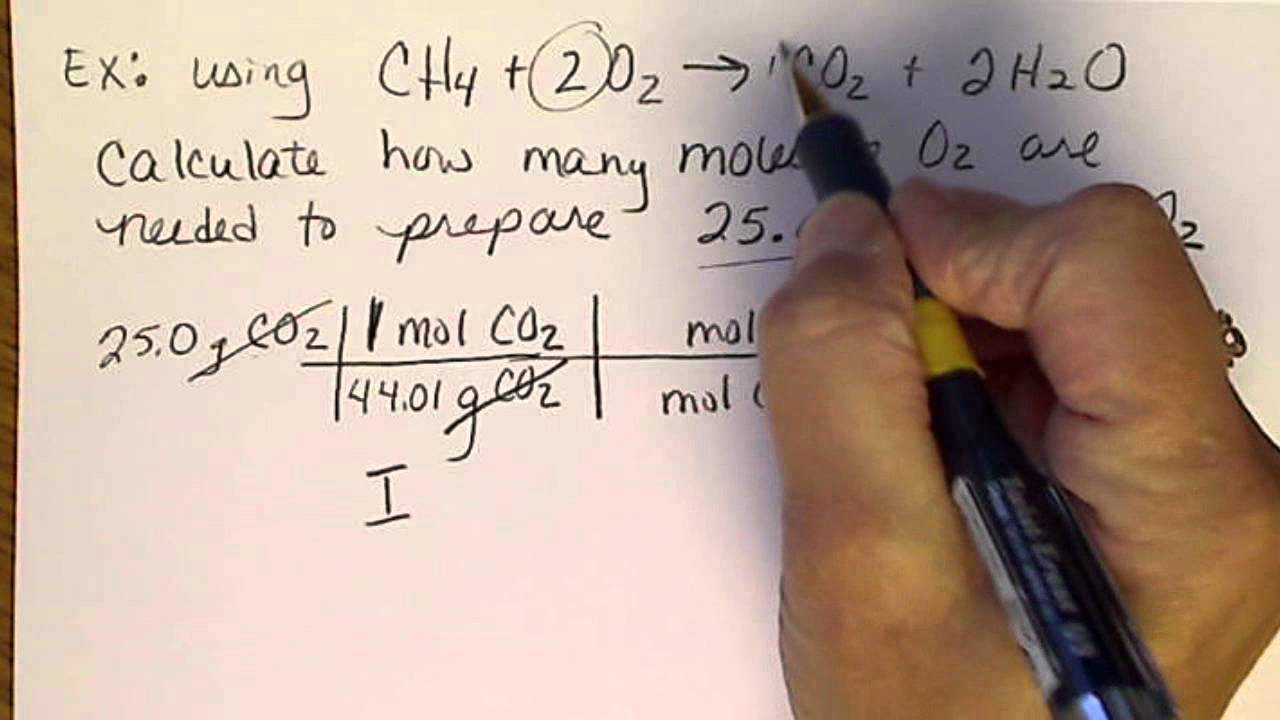
Stoichiometric Calculations: An example of doing 2 (out of 3 steps

Stoichiometry with Mass - YouTube

Stoichiometry: Mass to Mass - Practice - 3 - YouTube

17 stoichiometry

Stoichiometry Problem Type 3: Mass to Mol | Science, Chemistry | ShowMe

Stoichiometry - IGCSE Chemistry | Free Exam Academy
.PNG)
Using Stoichiometry to Determine a Formula
